The treatment – contraceptive pills and patches

Contraceptive pills either contain oestrogen and progestogen, (combined oral contraception or COC), or are progestogen only pills. Combined hormonal contraception (CHC) comprises the COC together with newer, longer-acting alternatives: the combined transdermal patch, Evra, and the vaginal ring (Nuvaring, not discussed here). CHC offers contraception, good cycle control and a wide-range of additional benefits including management of heavy menstrual bleeding, dysmenorrhoea, endometriosis, acne, PCOS, PMS and premature menopause. But CHC has significant contraindications4, which are summarised in the UK Medical Eligiblity Criteria for Contraception Use (UKMEC) guidelines.5
Few medical conditions restrict the use of the progestogen only pills 6, so this can be safely used in many conditions where oestrogens are contraindicated. Unscheduled bleeding is common with progestogen only pills and the major reason for women stopping this method.6 progestogen only pills are classified as traditional progestogen only pills or desogestrel-containing formulations. Desogestrel pills may be more effective but traditional formulations are more likely to give a regular bleeding profile, which may be preferable to women.
CHC and desogestrel-progestogen only pills work mainly by inhibiting ovulation, whereas the primary mechanism of traditional progestogen only pills is thickening cervical mucus.4,6 These different modes of action account for the different time taken for the contraceptive effect to be achieved and sustained. This is reflected in the ‘how to start’ and ‘missed pills’ advice. CHC and progestogen only pills can be started on day 1-5 of the menstrual cycle with no need for additional precautions (e.g. barrier methods).
When starting COC after day five of the cycle, additional contraception is advised for seven days compared to 48 hours when starting the progestogen only pills. A ‘missed’ COC pill is one more than 24 hours after it was due whereas traditional progestogen only pills are missed after only three hours and desogestrel progestogen only pills after 12 hours. No additional precautions are needed when switching between these methods unless changing from a traditional progestogen only pills , when condoms must be used for seven days. Missed pill guidance varies between different formulations but when doing an initial ‘pill teach’ with patients, the following simplified common rules can be used:
- Take the last missed pill as soon as remembered
- Take the next one when it was due (even if that means taking two in one day)
- Continue to take future pills as usual
- Use condoms for seven days (CHC) or 48 hours (progestogen only pills )
- If you’ve had sex and are unsure what to do, seek advice regarding emergency contraception and bring your pill packets with you if possible
Standard current treatment
The COC is conventionally prescribed as 21 days of pill taking followed by a seven day pill-free interval. Everyday formulations with seven placebo pills are also available and can be helpful for women who find compliance difficult. progestogen only pills are taken daily with no break. The patch has the advantage of needing to be changed weekly rather than daily and is also typically prescribed with three consecutive week patches followed by one-week break. Ethinylestradiol is the oestrogen used in most COCs because of its high oral bioavailability in combination with many alternative progestogens. Evra is a 4.5cm2 patch that contains 600mcg ethinylestradiol and 6mg norelgestromin.
Before initiating pills or patches, the patient should be advised of the mechanism of action, efficacy, benefits, risks and side effects. Medical and family history should be taken, enquiring specifically about migraine, VTE risk factors and breast cancer. A drug history identifies significant interactions e.g. enzyme-inducing medication such as carbamazepine and St John’s wort, which reduce efficacy of pills and patches (non-enzyme inducing antibiotics do not reduce effectiveness).7 Blood pressure and BMI should be recorded and patients should be given written information. Follow up should be at three months and then annually.
When starting COC, choose a preparation with a mid-dose i.e. 30mcg ethinylestradiol, and a progestogen associated with the lowest increased risk of VTE e.g. Microgynon 30 or equivalent. The formulation of the pill can then be tailored according to any side effects (see table 1). Lower oestrogen pills can be considered in the event of nausea or headaches and higher oestrogen pills can be considered if cyclical symptoms or breakthrough bleeding are prominent features.8 An alternative progestogen is advisable if side effects such as sore breasts or bloating predominate. Dianette (containing cyproterone acetate) and drosperinone-containing formulations (e.g.Yasmin) have anti-androgenic properties that can be helpful in the context of acne or hirsuitism.9
Since drosperinone is a spironolactone analogue with mild diuretic effect and anti-aldosterone properties, Yasmin can also be used to good effect if bloating or PMS are prominent.10 Users of the patch, which avoids first pass metabolism, have been seen to experience more systemic side effects such as breast discomfort, dysmenorrhoea, nausea and vomiting than pill users11 and there is potential for local skin irritation.
| Oestrogen | Progestogen |
|---|---|
|
Nausea |
Breast tenderness |
|
Bloating |
Bloating |
|
Headaches |
Headaches |
|
Dyspepsia |
Acne |
|
Leg cramps |
Greasy hair |
|
Ovarian cysts |
|
|
Mood change |
|
|
Loss of libido |
Table 1: Side effects of oestrogen and progestogen
What’s newly available?
There is a wide and rapidly changing range of pills, with many different brands containing identical formulations. For example, there are 14 different brands of identical desogestrel pill marketed in the UK, ranging in cost between £1.93 and £9.55 for a three month supply.12 Similarly, a typical first-line COC containing 30mcg ethinylestradiol and 150mcg levonorgestrel is available as eight different brands ranging from £1.80 to £29.23. Generic prescribing is therefore the key to facilitating minimum cost for exactly the same preparation. Contraceptive pills and the patch are also now widely available to patients via online pharmacies.
New COCs include Qlaira (oestradiol valerate and dienogest) and Zoely (oestradiol hemihydrate and nomegestrol) with formulations more similar to natural oestrogen and progestogen. These have been designed for a shorter lighter bleeding profile and theoretical safety advantages. Qlaira is a quadriphasic pill (four different hormone doses taken sequentially) whereas Zoely is a constant dose, monophasic pill. Qlaira and Zoely are licensed to use continuously,13 with Qlaira having 26 active pills and two placebo pills and Zoely a 24/4 dosing regimen. But both are expensive preparations. Pills containing drospirenone and 20mcg ethinylestradiol are also now available e.g. Daylette, Eloine. These contain the same progestogen component as the 30mcg ethinylestradiol Yasmin COC but have a 24/4 dosing regimen.
A new lower dose patch, Lisvy, containing 550mcg ethinylestradiol and 2.1mg gestodene has a European license and is awaiting UK approval.14 This is equivalent to a 20mcg ethinylestradiol pill whereas Evra is equivalent to a 35mcg pill. The patch is smaller, round and transparent which may have additional advantages for placement as well as the advantage of lower risk of systemic side effects.
What has fallen out of fashion and why
Recently, there has been media attention related to two major safety concerns: VTE and depression. VTE in women of reproductive age is rare15 and while use of the CHC increases VTE risk, this remains a low absolute risk. The increase in VTE risk is dependent on the type of progesterone and the Evra patch may be associated with a slightly higher risk.15 Co-cyprinol (Dianette) is also no longer recommended for solely contraception14 due to VTE risk but is licensed for acne that is refractory to antibiotic treatment. The risk of VTE is much lower than that associated with pregnancy and the postpartum period15 – see figure 1. It is estimated that 1% of VTE associated with CHC is fatal.16
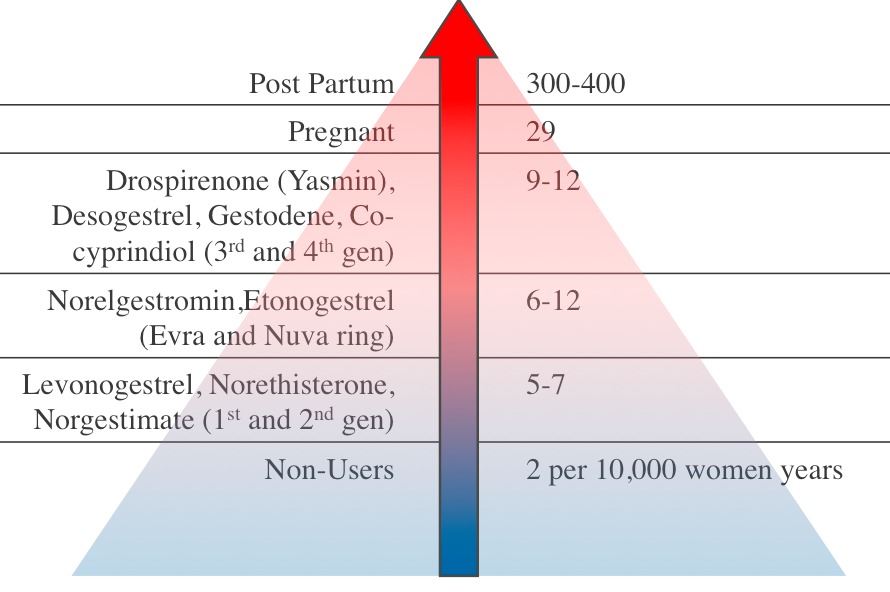
Figure 1: Relative VTE Risk
In most women benefits of CHC outweigh the risks, but all women should be assessed for other VTE risk factors such as obesity, smoking, immobility and personal or family history of VTE or thrombophilia. CHC should be avoided if significant VTE risk factors are present. Women should be fully informed of the potential risks and encouraged to be vigilant for symptoms of VTE.
A recent large prospective cohort study17 conducted in Denmark found users of hormonal contraception were more likely to be diagnosed with depression and prescribed antidepressants. But this study was not able to prove causation and there were many possible confounding factors. Further research is needed before changes in clinical practice. Other evidence with regard to potential increased risk of stroke, cardiovascular disease and breast cancer again show that the absolute risks remain small in premenopausal women. Any small increased risk of breast or cervical cancers associated with CHC is likely to reduce after stopping.
Prescribers are advised to refer to the UKMEC5 when evaluating risk factors, but it should be remembered that the UKMEC applies to prescribing related to contraceptive usage rather than for medical indications. Specialist advice from the local consultant in sexual and reproductive healthcare can be sought if there are multiple or complex safety concerns and risk should be balanced against that of unplanned pregnancy. CHC is also associated with health benefits such as decreased risks of ovarian, endometrial and colorectal cancers4 and COC has been associated with a reduction in all-cause mortality and no overall increased risk of cancer compared to never users.18
There have been concerns relating to prescribing for obese women. Past practice of prescribing double dose progestogen only pills to women with elevated BMI is not
recommended and there is no evidence to show increased risk of pregnancy in progestogen only pills or COC users with higher BMI.4,6 The summary of product characteristics for the patch states that efficacy may be decreased for women weighing >90kg and advises an alternative method. Progestogen only pills are usually a safer option than CHC in women with a BMI >35kg/m2.
Phasic pills, which vary the amount of hormone through the cycle, were introduced in an attempt to mimic the physiological pattern. But these have shown no evidence-based advantage over monophasic pills in relation to adverse side effect profile or cycle control.19,20 The majority of COCs are now monophasic.
Special/atypical cases and their treatment/tailored treatment
Tailored pill taking and extended regimens are becoming much more popular ways of prescribing the COC or patch. This involves bi- or tri-cycling i.e. omitting the pill-free week for one or two months and then taking a four or seven day break, or continuous use until three consecutive days of bleeding and then a four day break. There is good data to show safety and satisfaction of continuous use of COC for up to 12 months.21 These regimens are still off license but may offer benefits in efficacy and reduction in bleeding and symptoms that occur in the pill-free interval e.g. in endometriosis.
It has become normal practice to ‘quickstart’ the pill or patch i.e. start at the time of request rather than wait for the beginning of a period, including starting immediately after emergency contraception. However, if ulipristal acetate (EllaOne) is used, hormonal contraceptives should not be started for at least five days and barrier methods or abstinence advised until hormonal contraceptive cover has been achieved (usually seven days).22 This is because ulipristal acetate is a selective progesterone-receptor modulator and therefore carries a theoretical risk that it may reduce the efficacy of hormonal contraception and vice versa. If quickstarting hormonal contraception is desired, then levonorgestrel emergency contraception should be used rather than ulipristal acetate.
Alternative contraceptive options
It is good practice to counsel all women requesting contraception about the availability of LARC methods, which are more effective and cost effective.23 These can be especially useful in the context of women with significant risk factors for VTE, other medical contraindications to CHC or drug interactions. There are also many barrier methods of contraception available, including a new flexible contraceptive diaphragm, Caya.24
Dr Annette Thwaites is a specialist registrar in sexual and reproductive health at Lewisham and Greenwich NHS Trust.
Dr Jane Dickson is a consultant in sexual and reproductive health at Oxleas NHS Foundation Trust.
Dr Helen Munro is a locum consultant in sexual and reproductive health at Hywel Dda University Health Board.
References
- Contraception. 1999 Jan;59(1 Suppl):11S-16S. Introduction of the pill and its impact. Tyrer L.
- National Survey of Sexual Attitudes and Lifestyles 2010-2012 (Natsal-3)
- Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D, editors. Contraceptive technology. 20th revised. New York: Ardent Media; 2011
- Combined Hormonal Contraception Clinical Effectiveness Unit FSRH October 2011 (Updated August 2012)
- Faculty of Sexual & Reproductive Healthcare. UK Medical Eligibility Criteria for Contraceptive Use (UKMEC). 2016
- Progestogen-only Pills Clinical Effectiveness Unit FSRH March 2015(Updated January 2016)
- FSRH Clinical Guidance: Drug Interactions with Hormonal Contraception. January 2017
- Gallo MF et al. 20microgram versus >20 microgram estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev 2011. CD003989
- Batukan C, Muderris II et al. Comparison of two oral contraceptives containing either drospirenone or cyproterone acetate in the treatment of hirsutism. Gynecol Endocrinol. 2007 Jan;23(1):38-44.
- Lopez LM, Kaptein AA, Helmerhorst FM; Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012 Feb 15 2:CD006586. doi: 10.1002/14651858.CD006586.pub4.
- Lopez L, Grimes D, Gallo M, Schultz K. Skin patch and vaginal ring versus combined oral contraceptive for contraception. Cochrane Database Sys Review 2010;(3):CD003552
- Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press. http://www.medicinescomplete.com> [Accessed on [23/02/2017]
- CEU Statement/Review Estradiol/Nomegestrol Combined Pill, Zoely May 2013
- Lisvy Summary of Product Characteristics http://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA1330-019-001_22072015155042.pdf
- Statement from the Clinical Effectiveness Unit Combined hormonal contraception and venous thromboembolism December 2016
- Hedenmalm K, Samuelsson E. Fatal Venous thromboembolism associcated with different combined oral contraceptives: a study of incidences and potential biases in spontaneous reporting. Drug Saf 2005,28.907-917
- Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of Hormonal Contraception With Depression. JAMA Psychiatry. 2016 Nov 1;73(11):1154-1162. doi: 10.1001/jamapsychiatry.2016.2387.
- Hannaford et al. Mortality among contraceptive pill users:cohort evidence from RCGP’s Oral contraception Study. BMJ 2010;340:c927
- Van Vliet HAAM, Grimes DA, Helmerhorst FM, et al. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
- Grimes DA, Lopez LM, Schulz KF et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2009, Issue 2.
- Edelman et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception (Review). Cochrane Database Systematic Review 2014; 3: Art. No.: CD004695
- FSRH Clinical Effectiveness Unit (CEU) Faculty of Sexual and Reproductive Healthcare (FSRH) response to new data on quickstarting hormonal contraception after use of ulipristal acetate 30mg (ellaOne®) for emergency contraception Sept 2015.
- National Institute for Health and Clinical Excellence. Long-acting reversible contraception. NICE guideline (CG30). 2005 (Updated September 2014)
- Faculty of Sexual & Reproductive Healthcare. New Product Review from the Clinical Effectiveness Unit One size contraceptive diaphragm (Caya®). August 2014
Pulse October survey
Take our July 2025 survey to potentially win £1.000 worth of tokens

Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.









