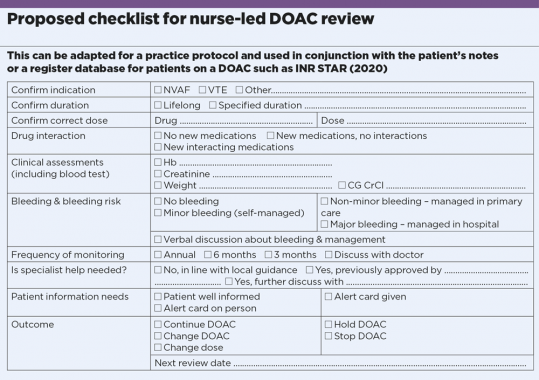
1. Confirm DOACs indication
The two licensed indications for DOAC are non-valvular atrial fibrillation and venous thromboembolism. All other indications should have been confirmed by a specialist. DOACs are contraindicated in moderate to severe mitral stenosis, metallic valves, anti-phospholipid syndrome and severe renal impairment (CKD5/creatinine clearance less than 15ml/min). The Medicines and Health products Regulatory Agency is a good source for updates.
2. Confirm duration
Treatment for NVAF is usually lifelong unless contraindications or bleeding complications develop. If the DOAC is stopped, documentation is required with plans for further review. If there is an absolute contraindication, there should be documented confirmation that no further anticoagulation is intended. In VTE, the specialist should specify the duration of treatment. Provoked VTE usually requires time-limited treatment, as in the NICE clinical knowledge summary.
3. Confirm correct dose
Several audits have demonstrated a high rate of incorrect DOAC dose for NVAF. The standard dose should be used by default, and reduced only where the summary of product characteristics criteria are met. Dose reduction because of high bleeding risk is not associated with any benefit. Renal function for dose adjustment should be assessed by calculation of Cockroft-Gault creatinine clearance (CG CrCl).
To ensure the patient is on the correct dose, check the EMC website or the BNF.
4. Check for drug interactions
A standard medication check should be undertaken to ensure no drugs with significant interaction have been started. In some cases, the DOAC dose will need to be reduced – for instance, edoxaban should be reduced with dronedarone, erythromycin, ciclosporin or ketoconazole. When adding a new drug for patients on a DOAC, interactions should be reviewed as suggested in the EHRA practical guide.
5. Remember blood testing requirement
A blood test is required at each review to include FBC, LFT, U&E, CG CrCl with the patient’s current weight. Ideally, the bloods should be scheduled a few days before so that the results are available for the review.
6. Remember bleeding risk
Direct questioning for bleeding is required. NICE guidelines encourage use of an objective bleeding risk score (HAS-BLED) to quantify the bleeding risk, and this should be updated and documented at each presentation. Patients should be told what to do about minor and non-minor bleeding.
7. Monitor at least annually
EHRA guidance advises that DOAC monitoring should roughly follow NICE CKD guidelines. Standard minimum is annual, and more frequently if there is increasing renal impairment and higher bleeding risk.
8. Consider specialist input
If local eligibility criteria are not met, it is important to confirm ongoing DOAC use with a specialist. In our region, haematologist approval is required for extreme body weight (under 40kg or over 120kg), CKD4 (CrCl 15-30ml/min) or unlicensed use in individual circumstances.
9. Don’t forget patient advice
The relative ease of use and stable dosing of DOACs (compared with warfarin) may falsely reassure patients and clinicians. But DOACs are potent and increase bleeding risk, and care must be taken to avoid injuries and manage any bleeding. Patients should carry an anticoagulant alert card at all times. Give them a practice phone number for queries.
10. Clarify outcome and follow-up
A DOAC review should end with a definitive outcome –continue DOAC, change dose, change anticoagulant, stop anticoagulant temporarily (and review after specified period), stop anticoagulant permanently. The next follow-up date should be confirmed. There should also be a safety-net system to recall patients due for reviews.
Dr Amit Mistri is a consultant in stroke medicine at Leicester Royal Infirmary (UHL) and Reena Patel is practice nurse at Willows Health, Leicester
Pulse voluntary donation scheme
Since the outbreak of this pandemic, Pulse has strived to support you, whether it be through our resources page, our ‘Clinical Crises’ series, holding policymakers to account with exclusives such as practices being supplied with faulty masks, or GPs being told to stop routine services in the hardest hit areas.
However, good journalism cannot be done on the cheap and, like the whole publishing industry, we have been affected by the economic slowdown. We also strongly believe the content we produce should remain free as we feel it is essential for you. Because of this, we have set up a voluntary donation scheme. There is no compulsion whatsoever to donate. But if you feel we are helping you, and you would like to support us, anything you can spare would be greatly appreciated. Read more here.
Pulse October survey
Take our July 2025 survey to potentially win £1.000 worth of tokens

Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.