What GPs need to know about the new Covid-19 therapeutics

Dr Toni Hazell outlines key information for GPs on the new Covid-19 treatments that are coming available for patients in the community
Up to now there have been two main options for the primary care management of patients with Covid-19. Patients have either been judged sick enough to be admitted, a decision usually made by video or ‘hot hub’ appointment depending on local arrangements, or they have been considered well enough to stay at home.
Patients in the latter group have been managed in a similar way to those with other viral upper respiratory tract infections, sometimes with oxygen saturation monitoring; we have had nothing extra in the way of pharmaceuticals.
All this may be about to change with the roll out of neutralising monoclonal antibody (nMAB) drugs and antiviral treatments for non-hospitalised patients.
The new treatments announced so far are sotrovimab (brand name Xevudy) and the combination casirivimab/imdevimab (Ronapreve), both given intravenously, and the antiviral molnupiravir (Lagevrio) which is taken orally.
The NHS has recommended these drugs should be made available for treatment of certain high-risk patients with Covid-19 in the community.1 In addition, a wider group of patients with Covid-19 may be offered molnupiravir as part of a trial.2
GPs will not prescribe the drugs directly but will refer patients to local hubs for the treatments; they are also encouraged to help recruit patients to participate in the trial.2
Change to policy position
Please note: since the original publication of this article NHS England has advised that the community policy for nMAB roll-out now focuses on sotrovimab only.
This is because of doubts over the efficacy of the nMAB casirivimab/imdevimab against the Omicron variant. By contrast, preliminary data indicate sotrovimab is effective against Omicron.
Casirivimab/imdevimab is still being used in hospitalised patients where the Delta variant is still prevalent, but in the community it is likely to be compromised against the increasingly prevalent Omicron variant.
The policy positions are published via the Central Alerting System here.
What are the new drugs?
Casirivimab/imdevimab (Ronapreve)
The nMABs casirivimab and imdevimab both bind to the spike protein of SARS-CoV-2, to prevent the virus from entering host cells and replicating.
The combination casirivimab/imdevimab is given by intravenous or subcutaneous infusion, within seven days of symptom onset. Initial evidence shows that Ronapreve reduces the composite outcome of admission or death by 70% and speeds recovery from Covid-19 symptoms by four days.
Casirivimab/imdevimab has been approved by the Medicines and Healthcare Products Regulatory Agency (MHRA) for use in prophylaxis and treatment of acute Covid-19 infection in adults and children aged 12 years and over. 3 However, as above, it is likely to be less effective against the Omicron variant and is not currently the focus for treatment of patients in the community.
Sotrovimab
Sotrovimab is a dual-action nMAB that both blocks viral entry into healthy cells and clears cells infected with SARS-CoV-2. It is given intravenously, within 5 days of symptom onset. The recommended dose is 500mg to be administered as a single intravenous infusion.1
One trial found a single dose of sotrovimab reduced the relative risk of hospitalisation and death by 85% when given to high-risk, unvaccinated non-hospitalised adults with mild-to-moderate Covid-19.1,4
It was approved early in December by the MHRA for the treatment of symptomatic adults and adolescents with acute Covid-19 who do not require oxygen supplementation and who are at increased risk of progressing to severe Covid-19 infection.5
Early pre-clinical data indicate sotrovimab is effective against the Omicron variant of SARs-CoV-2, according to the manufacturer GlaxoSmithKline.6
Molnupiravir – Second-line, to be used if not possible to use nMAB
Molnupiravir is a pro-drug which, after two steps of metabolism, becomes N-hydroxycytidine triphosphate (NHC-TP). NHC-TP is incorporated into viral RNA and causes an accumulation of errors in the genome which inhibit replication. It is most effective when taken in the early stages of infection.
Molnupiravir is given orally as a five-day course. Interim data from one trial showed a significant reduction in all-cause admission and death, from 14% with placebo to 7% with molnupiravir.7 However, more recent analysis of the full results suggests it is less effective than this, reducing the relative risk of admission and death by 30%.
Molnupiravir has been approved by the MHRA for treatment of mild to moderate Covid-19 in adults (aged 18 years and over) with a positive SARS-CoV-2 diagnostic test and at least one risk factor for developing severe illness.8
Which patients will be eligible for treatment?
Patients considered to be at the highest risk, who do not otherwise need admission, will be considered for treatment with nMAB in the community. A list of those considered to be at the highest risk is given in the box below.
Where an nMAB is contraindicated, not recommended or the administration of a nMAB is not possible, patients may be treated with a five-day course of molnupiravir, provided the onset of symptoms is in the last 5 days.1
The policy may change as further data emerge on the Omicron variant and any impact it has on the efficacy of treatments.
Patients eligible for treatment with nMAB or antivirals
The following high-risk patients will be eligible for treatment:
- Down’s syndrome and other genetic conditions that might reasonably be expected to reduce immune competence
- Sickle cell disease
- Solid cancers, or patients who have received radiotherapy within the last six months or chemotherapy within the last 12 months.
- Certain patients with a haematologic malignancy – eg, those within 12 months of a stem cell transplant, who have active graft vs host disease or who are within 3-6 months of various therapies.
- Renal disease including those with a transplant (or a failed transplant within the last 12 months), all those with CKD 4 or 5 and those who have had B cell depleting therapy in the past 12 months or are otherwise immunosuppressed.
- Liver disease including cirrhosis, transplant and immunosuppression due to therapy for liver disease.
- Immune mediated inflammatory disorders, including immunosuppression due to medication
- Primary immune deficiencies
- HIV or AIDS where the viral load is high or the CD4 count is <350 cells/mm3 (or > cells/mm3 that with additional risk factors)
- Recipients of solid organ transplants not covered in other categories
- Neurological conditions – multiple sclerosis, motor neurone disease, myasthenia gravis and Huntington’s disease.
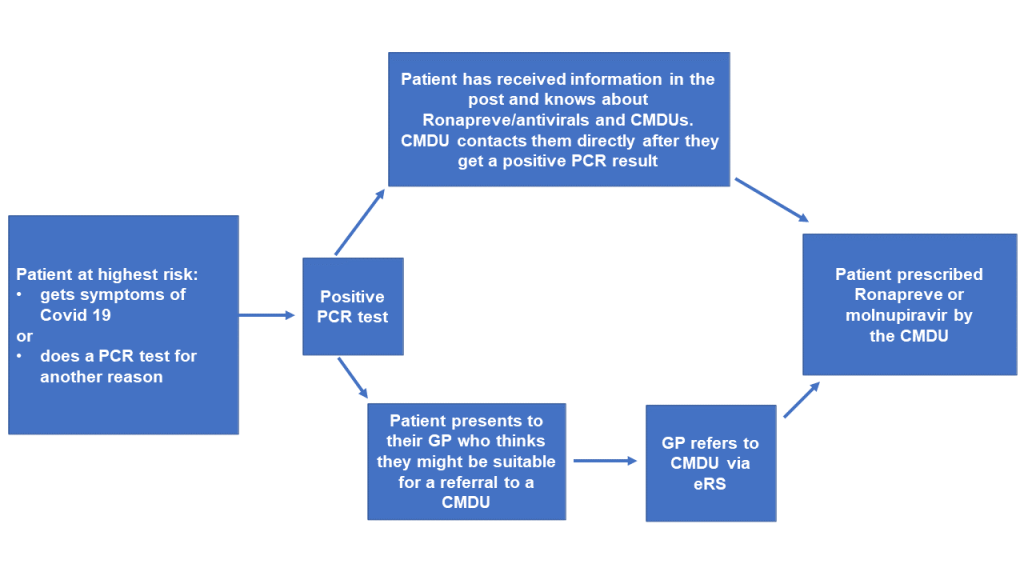
How can those at the highest risk access treatment?
A policy for access to Casirivimab/imdevimab or antivirals on the NHS for non-hospitalised patients, effective in all four UK nations, was published on 8 December to be effective from 16 December. A policy for access to sotrovimab was subsequently published, effective from 20 December.1
Locally commissioned Covid Medicines Delivery Units (CMDUs) have been asked to prescribe and administer nMABs to patients in the highest risk group who have a diagnosis of Covid-19 proven on a PCR test.
For sotrovimab, Covid-19 must have been confirmed with the previous 5 days, with symptom onset no more than five days previously.
For initiation of casirivimab/imdevimab Covid-19 must have been confirmed by PCR done within the last 72 hours, with symptom onset not more than seven days previously.
Patients aged 12 years or over can be seen at a CMDU. If nMABs are contraindicated (likely only to be because of known allergy to a constituent) or cannot be given for another reason, then those aged 18 or over can be given molnupiravir, if the onset of symptoms is in the last 5 days.
Trusts and CCGs are in the process of writing to patients in the highest risk group (by letter or email) so that they know that they may be eligible for treatment in a CMDU if they test positive for Covid-19.9 This cohort will also be sent a PCR test to keep at home so that they can do the test rapidly if they develop symptoms.
If they test positive, the local CMDU should contact them directly to discuss and arrange treatment.
However, the pandemic and introduction of the shielded patient list uncovered some of the limitations of coding and it is likely that some patients will not receive this letter or email, and that there will be some PCR tests that do no match directly to a patient’s health record.
Under these circumstances (if they haven’t heard anything within 24 hours of their positive test) patients are being told to contact their GP practice (or 111, out of hours) for an urgent referral to a CMDU.
GPs can refer to the CMDU via the e-RS system, where they are listed under the specialty of infectious diseases.
At this point in time GPs should not be prescribing nMABs or molnupiravir.
Which patients will receive molnupiravir as part of the trial?
As well as the use of CMDUs for the very highest risk patients, molnupiravir will be available to a broader cohort of patients via the Platform Adaptive trial of NOvel antiviRals for eArly treatMent of Covid-19 In the Community (PANORAMIC) trial which is starting this month, funded by the National Institute for Health Research and led by the University of Oxford.10 Practices and individual patients can sign up to take part – eligibility criteria are listed in the box below.
Patients eligible for PANORAMIC trial
Patients eligible to participate include those with:
- Covid-19 symptoms which have begun in the last five days and
- Positive PCR test for Covid 19 and
- Aged 50 and over or aged 18-49 with a pre-existing condition. Pre-existing conditions are as follows:
- Respiratory disease (COPD, cystic fibrosis, asthma requiring at least daily medication)
- Chronic heart or vascular disease
- Chronic kidney disease
- Chronic liver disease
- Chronic neurological disease (eg, dementia, stroke, epilepsy)
- Down’s syndrome
- Type 1 or 2 diabetes
- Immunosuppression due to disease or treatment
- Recipient of a transplant (solid organ, bone marrow or stem cell)
- Obesity with a BMI >35
- Severe mental illness
- Resident of a care home
- Judged by the recruiting clinician or research nurse to be clinically vulnerable
Full details of eligible patients are available in Appendix 1 of the NHS policy document here.
How can GPs put patients forward for the trial?
The last of the criteria suggests that GPs may have some influence on those who can join the trial – recruiting clinician is defined as a ‘registered medical practitioner or trained study nurse’, which would presumably include a GP. The list of pre-existing conditions already given is, however, wide and it seems unlikely that there will be many patients who are not in one of these categories but considered by their GP to be clinically vulnerable. A GP approached by a patient wanting to enter the trial but without a listed condition might reasonably feel nervous about making this decision themselves, in which case the patient could be signposted to the trial website to be assessed by a study nurse.
Study participants will be randomised by computer to usual care or usual care plus five days of molnupiravir 800mg twice a day, which will be delivered in the post and taken at home. They will be asked to complete an online diary of symptoms and medical care received for 28 days (trial staff will collect this information by phone for those with no internet access) and will be contacted at three and six months and asked about long-term symptoms of Covid-19.10 PANORAMIC is a platform study and there is the potential for other antiviral drugs to be added to it in the future, which may be taken for a different length of time. There is no placebo arm, the control being usual NHS care rather than a placebo antiviral.
What are the side effects of these treatments? Are there any contraindications?
Casirivimab/imdevimab (Ronapreve)
Side effects of casirivimab/imdevimab appear to be mild – one in ten people will get a reaction at the injection site and less common reactions include dizziness, nausea, hot flushes and chills.
It is thought that one in 10,000 people will have an anaphylactic reaction to casirivimab/imdevimab and the only contraindication is a known allergy to one of the ingredients.
All the data so far have been obtained in people who weigh at least 35.5kg so caution may be needed for those under this weight; this is unlikely to be an issue for the majority of the adult population.
Regarding pregnancy, the patient information leaflet11 says that it would only be given if ‘the potential benefits of treatment outweigh the potential risks to the mother and the unborn child’ – this is likely to be a decision to be made on a case-by-case basis by CMDUs.
Sotrovimab
Hypersensitivity reactions, including serious and/or life-threatening reactions such as anaphylaxis, have been reported following infusion of sotrovimab.12 The only contraindication is hypersensitivity to one of the ingredients, and drug interactions are considered ‘unlikely’.
According to the patient leaflet common side effects (affecting up to one in 10 people) are less severe allergic reactions, such as skin rash and itching.13
Sotrovimab may be used during pregnancy ‘where the expected benefit to the mother justifies the risk to the foetus’.12
Molnupiravir
The nature of a trial drug is that side effects are not always clearly established, but previous data suggest that side-effects of molnupiravir are likely to be mild and may include diarrhoea, nausea, dizziness, headache, rash and urticaria.4 Obviously there are no long-term data yet. If a patient on the trial reports a possible side-effect to their GP, it should be escalated using the yellow card system.13 No reduction in dose is needed due to age or reduced renal or liver function and there are no known drug interactions;4 molnupiravir is not an inhibitor or inducer of any enzymes or transporters involved in drug metabolism, so the potential for drug interactions is low. We have no experience of overdose and it is advised that this should be treated with supportive measures only.
Pregnant women are not included in the PANORAMIC trial and all women of childbearing age will be asked to do a pregnancy test before confirming their inclusion in the study. They will be asked to commit to using reliable contraception during the study and for four days after the last dose of molnupiravir. There is early animal data suggesting a risk of post-implantation losses and malformations of the eye, kidney and skeleton, although these occurred when the study animals were exposed to higher doses per kilogram than will be given to humans. No studies have been done on lactating animals and so for the moment molnupiravir is not recommended in breastfeeding women.
The Covid-19 pandemic has been a part of our lives for nearly two years and for most of that time, we in primary care have only been able to treat patients symptomatically, referring the sickest to secondary care. The PANORAMIC trial may usher in the start of a new era where Covid-19 becomes just another infection that we diagnose and treat in primary care as a matter of routine.
Dr Toni Hazell is a GP in North London
Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.
Related Articles
READERS' COMMENTS [7]
Please note, only GPs are permitted to add comments to articles










Classic example of how NHS fails patients and how the NHS is often a mechanism not to provide, but to restrict access to healthcare.
It is impossible to predict reliably who will get ill. Undoubtedly some who might have benefited will not have been offered treatment, rationing in action. The manufacturers could undoubtedly provide more doses, but those not eligible for NHS treatment will be denied the option of paying the cost of treatment themselves.
Quite possibly people will be dying to protect the “free at the point of care” ideology.
Very helpful article – thank you.
Entirely predictably, the letter to patients makes no sense and they are already ringing the practice to get it explained. Those that can get through of course, as the telephone is just a little busy at the moment.
Well done NHS England, another triumph you useless f###**s.
Really useful Toni – thank you !
It seems a little strange that the PANORAMIC trial is not using placebo in the control arm.
Given that the outcomes measured appear to be self-reported, there is high likelihood of confounding by the placebo effect which could easily have been solved by a blinded placebo trial.
As it is they are effectively asking one group of patients “how do you feel having been denied any treatment” and the other group “how do you feel now you’ve had our amazing wonderdrug?”
very strange severe lung disease not included on list.
Very obvious that not enough people are getting these drugs. Some who should have done will die.