NHS launches Wegovy prescribing for weight-loss after securing limited stock
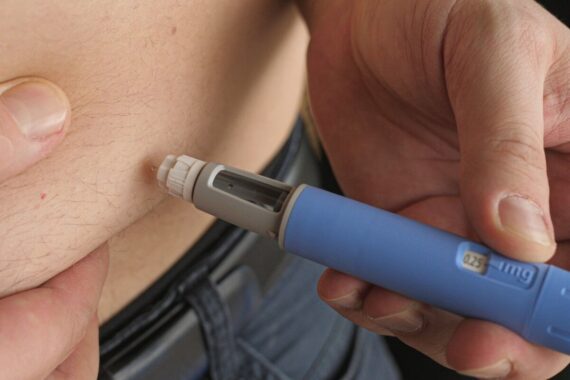
Semaglutide, marketed as Wegovy, will become available via the NHS as a weight-loss drug from today, after a limited stock was earmarked to fulfil new NICE guidance.
Manufacturer Novo Nordisk announced that Wegovy will be available in specialist NHS weight management services for people who meet the NICE criteria, or privately through a registered healthcare professional, through a ‘controlled and limited launch’.
NHS England said it had secured sufficient stock for 50,000 patients to start obesity treatment with help from the injections.
Semaglutide is already prescribed in the NHS for diabetes treatment however GPs have been advised not to start new patient on the drug since last year, due to a global supply shortage caused by off-label prescribing for weight loss.
In March, NICE recommended Wegovy for use as part of a patient’s treatment for obesity for the first time, in an NHS specialist weight management service and with the support of a multi-disciplinary team.
More recently, it proposed that four digital apps could be used for weight management support and even prescribing of weight-loss drugs.
NICE has said semaglutide should be prescribed to adults who have at least one weight-related comorbidity and a BMI of at least 35kg/m2, alongside a reduced-calorie diet and increased physical activity.
In a statement, Novo Nordisk said: ‘As we expect supply to be constrained for the foreseeable future, a proportion of available supply will be allocated for use only within the NHS to allow healthcare professionals to implement NICE guidance.
‘We will continue to work with healthcare professionals to help ensure that patients with the highest unmet medical need are prioritised.
‘We are closely monitoring Wegovy demand and are working with regulators and providers to ensure people living with obesity can have access to and remain on treatment.’
An NHS England spokesperson told Pulse: ‘Despite global supply constraints, NHS England has successfully secured sufficient supply of Wegovy to meet expected patient demand and implement NICE guidance.
‘Around 50,000 eligible patients in England will be prescribed Wegovy through NHS specialist weight management services, that are able to provide appropriate multidisciplinary care.’
The Government has said it is looking at how GPs could safely prescribe obesity drugs, as part of plans to reduce pressure on the NHS and cutting waiting lists, with a two-year pilot backed by up to £40m to ‘explore ways to make obesity drugs accessible to patients living with obesity outside of hospital settings’.
However the BMA warned that GP workload would need to be taken into account once the scheme was rolled out more widely.
Last month, Novo Nordisk claimed Wegovy could also reduce risk of cardiovascular events by 20%, as it announced headline findings from its SELECT trial, although they have not yet been published in a peer-reviewed journal.
Experts said that while the top line results of the trial sound promising, more details are needed on the trial to give it proper consideration, including the examination of safety aspects.
It comes as patients with type 2 diabetes on semaglutide and other glucagon-like peptide-1 receptor agonists (GLP-1 RA) drugs are having to stop taking them, amidst ongoing worldwide shortages caused by off-label prescribing for weight loss.
The RCGP has warned that unregulated use of semaglutide is posing ‘a genuine threat to patient safety’.
NICE recommendations for Wegovy
1.1 Semaglutide is recommended as an option for weight management, including weight loss and weight maintenance, alongside a reduced-calorie diet and increased physical activity in adults, only if:
- it is used for a maximum of 2 years, and within a specialist weight management service providing multidisciplinary management of overweight or obesity (including but not limited to tiers 3 and 4), and
- they have at least 1 weight-related comorbidity and:
- a body mass index (BMI) of at least 35.0 kg/m2, or
- a BMI of 30.0 kg/m2 to 34.9 kg/m2 and meet the criteria for referral to specialist weight management services in NICE’s guideline on obesity: identification, assessment and management.
- the company provides semaglutide according to the commercial arrangement.
Use lower BMI thresholds (usually reduced by 2.5 kg/m2) for people from South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean family backgrounds.
1.2 Consider stopping semaglutide if less than 5% of the initial weight has been lost after 6 months of treatment.
1.3 These recommendations are not intended to affect treatment with semaglutide that was started in the NHS before this guidance was published. People having treatment outside these recommendations may continue without change to the funding arrangements in place for them before this guidance was published, until they and their NHS clinician consider it appropriate to stop.
Source: NICE
Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.
Related Articles
READERS' COMMENTS [2]
Please note, only GPs are permitted to add comments to articles










What?———–no training
Perhaps a small charge for those using this service?
If someone can afford the food to achieve and maintain a BMI of 35+ then there are definitely savings to be made from eating less.