‘Significant’ changes to childhood vaccination schedule announced
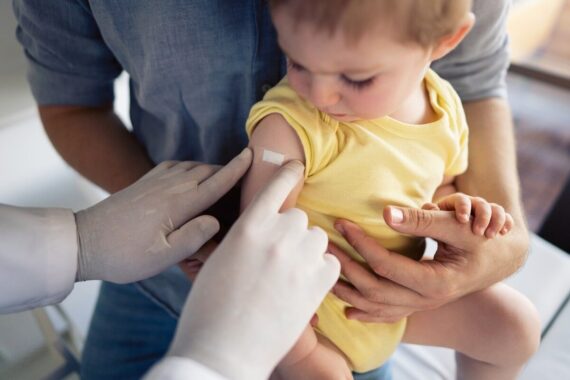
The Government has announced a raft of ‘significant’ changes to the childhood vaccination schedule, including a new routine appointment at 18 months.
Recommended shifts in a number of vaccination timings, the first of which will come into force in July, have been triggered by the discontinuation of the Mentorix Hib/Men C vaccine that until now has been offered in children turning a year old.
With no other Hib/Men C vaccine on the market, the Joint Committee on Vaccination and Immunisation (JCVI) has agreed that protection against meningitis C is no longer needed in this age group due to ‘excellent population control provided by vaccination of adolescents’.
Instead, a new fourth dose of the 6-in-1 vaccine that contains Hib alongside diphtheria, tetanus, pertussis, polio and hepatitis B will be introduced at 18 months from January 2026.
The second MMR dose will also be moved from three years to this new 18-month appointment to help improve uptake and provide earlier protection, the letter from the UK Health Security Agency and NHS England sets out.
JCVI first recommended the changes to the immunisation schedule in 2022 while noting that stocks of Mentorix would run out this year. Several options were considered.
An additional appointment at 18 months was approved because it also provides ‘the opportunity to strengthen the protection’ against polio, diphtheria and pertussis.
And the committee noted that studies in London and elsewhere had shown that where the second dose of MMR had been brought forward in response to outbreaks, it led to significantly higher coverage once children reached the age of five.
Once the changes are in place, the one-year appointment will still offer vaccination for the first MMR dose and meningitis B and pneumococcal booster jabs.
From July this year there are also changes to the vaccine schedule given at two, three and four months, after recommendations from the JCVI.
The second meningitis B vaccine dose will now be given at 12 weeks instead of 16 weeks after a recent clinical study showed the benefits of earlier protection against the infection.
To avoid an increase in injections given at the 12-week appointment, the first dose of pneumococcal vaccine (PCV13) will be moved to 16 weeks, the guidance sent to all those involved in commissioning and delivering vaccines states.
‘The short delay in PCV13 is unlikely to be significant due to excellent overall control of the serotypes covered by PCV13,’ the JCVI has advised.
Any children who were eligible for the selective neonatal Hepatitis B vaccination programme who would have been vaccinated at one year will no longer need to be because of the new 18-month jab.
The hepatitis B surface antigen blood spot test will still be needed in those who are eligible because of potential transmission in pregnancy but can be done at any time between one year and 18 months.
Most of the changes impact children born on or after 1July 2024, a table outlining the changes explains.
Professor Azeem Majeed, a GP and professor of primary care and public health at Imperial College London, said: ‘The changes to the NHS childhood vaccination programme have important implications for general practices and parents of children in England.
‘For general practices, the guidance requires significant adjustments to vaccination schedules, increased administrative efforts, and proactive communication to ensure compliance and maintain high uptake.
‘For parents, the changes mean adapting to a new 18-month appointment, understanding the revised schedule based on their child’s birth date, and ensuring timely vaccinations.’
He added the new 18-month appointment will mean additional staff time and resources.
Dr Julie Yates, deputy director immunisation programmes, implementation and clinical guidance at UKHSA, said: ‘Following a review of the latest evidence, JCVI recommended a number of changes to optimise the Childhood Immunisation programme and increase overall protection of children in the UK.
‘With the UK close to seeing an end to Meningitis C circulating, JCVI advised that a Men-C vaccination is no longer required for infants due to the excellent population protection provided by the adolescent Meningitis ACWY programme. And with the joint Hib-MenC vaccine now no longer being manufactured, a fourth Hib dose, which protects against Haemophilus influenzae type b – a bacteria that can cause life-threatening infections – will now be given at a new appointment at 18-months from January 2026.
‘Other changes, such as the bringing forward of the Meningitis B vaccine are based on evidence that will save the lives of more very young babies.
‘Phase one of the changes start in July and we are working closely with the NHS and other partners, including Royal Colleges, to ensure healthcare professionals delivering the routine programme are aware of the coming changes and have the resources and guidance needed to deliver them.’
Changes to routine childhood immunisation schedule
From July 2025
Cessation of routine Hib/Men C (Mentorix) in children turning one
Second meningitis B dose moved from 16 weeks to 12 weeks
First PCV dose moved from 12 weeks to 16 weeks
Cessation of monovalent hepatitis B dose offered at one year in selective neonatal Hep B programme
From January 2026
Introduction of an additional fourth dose of DTaP/IPV/Hib/HepB at new 18-month appointment
Second MMR dose moved from 3 years 4 months to 18 months.
Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.
Related Articles
READERS' COMMENTS [2]
Please note, only GPs are permitted to add comments to articles













All of this shuffling is irrelevant if they don’t remove childhood imms from QOF, or at least allow exception reporting.
Practices who don’t have a cat in Hell’s chance of hitting targets due to the rise of refuseniks will cease chasing down reluctant parents (as we did previously), and immunisation rates will continue to decline to dangerously low numbers.
This was a sadly predictable and totally avoidable disaster.
Just wish the child immunisation targets would allow parental refusal as allowing parental choice then penalising GPs with near impossible targets to hit makes no sense. Either manadate the vaccines with qualification for benefits or nursery places or allow opting out from parents, you can have neither and then penalise GP income for the allowing choice.
This confusing change to schedules will only make the facebook refusers even more adamant on vaccine safety concerns, and will only be stamped out as suggested above. Let them decline or make them mandatory