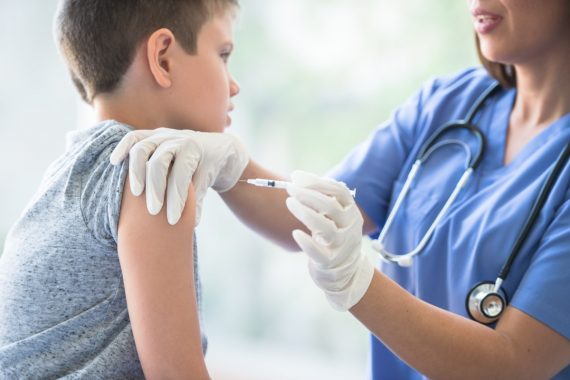
The Pfizer Covid vaccine could be administered to children aged five to 11 in the US within weeks.
The FDA’s vaccines advisory panel has recommended that the FDA grants emergency use authorisation for the jab, concluding that the benefits of having the jab outweigh any risks.
The committee reviewed evidence including Pfizer’s clinical trial, during which 4,500 children were given two doses of the jab 21 days apart. The results showed a safety profile of 90.7% in children who hadn’t previously been infected with Covid. The safety and effectiveness of the jab was consistent with what Pfizer has found in studies with adults, who receive three times the dose of what is being recommended for five to 11-year-olds.
Currently, the Food and Drug Administration (FDA) has approved emergency use of the vaccine for people 12 years of age and older.
The FDA and the Centers for Disease Control and Prevention (CDC) are expected to make a decision for giving the jab to children aged five and over within the next week. If the recommendation is approved, doses of the vaccine will be immediately shipped across the US.
The Pfizer study also looked at the vaccine in children aged six months to two years, and aged two to five, and the results for this are expected by the end of the year.
‘We appreciated the opportunity to present our clinical data demonstrating the safety and high efficacy of our COVID-19 vaccine in children five to under 12 years of age,’ said Kathrin Jansen, senior vice president and head of vaccine research and development at Pfizer,
‘Covid is an ongoing threat for the more than 28 million young children in this age group in the US, as they remain at risk for this infection. About 10% of all weekly US cases occur in children five to under 12 years of age with a potential risk of complications.
‘In addition, immunising children will help to get us closer to herd immunity, with the potential to stem the pandemic sooner.’
Pfizer and BioNTech have applied for their vaccine to be used on this age group to other regulators, including the European Medicines Agency. The UK is currently vaccinating children aged 12 to 15 with one dose.
Click to complete relevant paediatrics CPD modules on Pulse learning.
Pulse October survey
Take our July 2025 survey to potentially win £1.000 worth of tokens