Patients switched to lookalike generic drugs less likely to complain
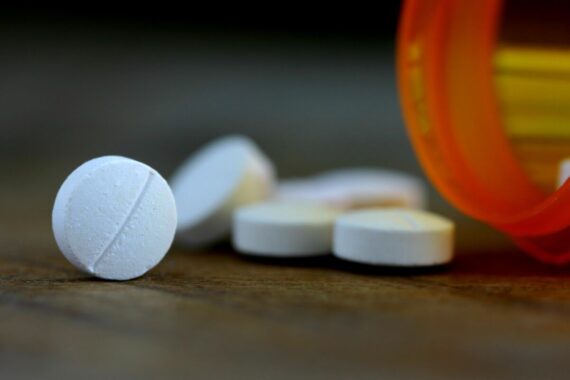
Patients whose branded drugs are replaced with a generic version that looks the same are less likely to report negative side effects.
A US study found that switch-back rates were 28% lower for patients who were switched onto an identical-looking authorised generic product.
Authorised products are classed as those which are identical in composition and appearance to the branded drug product. They are usually marketed by the brand manufacturer after the drugs patent has expired.
The observational cohort study looked at over 200,000 patient records across private and public insurance programs, comparing the frequency with which patients returned to the branded medicine after a generic swap.
They found that switch-back rates were consistently lower for patients who changed from a branded to an authorised generic product.
The paper, published in the BMJ, said changes in a drugs appearance ‘may induce the nocebo effect, defined as negative effects in treatment efficacy or tolerability mediated by negative expectations, through psychological or neurobiological factors’.
According to study author Dr Rishi Desai, an instructor of medicine at Harvard Medical School, the rate of patients being switched back to branded versions of drugs is frequent – at 8% annually – in a nationally representative US cohort.
He said: ‘It is unclear why doctors decide to switch back some of their patients, but some contributing factors could be a desire to ensure that the patients’ expectations for their care are met or a belief among physicians themselves that generics do not work as well as the brand.’
In order to overcome the trend, the authors suggest that more generic products should be manufactured to resemble the branded version.
The paper suggested that ‘courts would likely not allow brand name manufacturers to claim intellectual property protections over their products’ appearance to prevent their use by generic manufacturers’.
They also said that doctors could discuss the nocebo effect with patients prior to switching, in an attempt to reduce the switch-back rate by making the patient aware that the ‘branded and generic drug products are of equal quality, safety, and efficacy’.
In 2014 a different US study found that patients were much more likely to stop taking a generic cardiac medication if there was a change in shape or colour between prescriptions.
Reseachers observed that the odds of stopping a medication increased by over one third after a change in pill colour, and by two thirds following a change in the shape of the drug.
Pulse July survey
Take our July 2025 survey to potentially win £1.000 worth of tokens

Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.










