Guidance on selecting alternative glucose lowering therapies for patients with type 2 diabetes has been issued in response to ongoing shortages of semaglutide and other glucagon like peptide-1 receptor agonists (GLP-1 RA).
The Primary Care Diabetes Society updated their advice after the latest information on national shortages warned that while some GLP-1 RA drugs are expected to be unavailable or suffer intermittent supply issues until the end of 2024, alternative medicines had become available.
A medicines supply notification on 18 March said that while shortages continue for semaglutide, dulaglutide and liraglutide pre-filled injection pens of various strengths there was enough supply of other drugs could to support both new treatment and patients unable to get hold of existing medicines.
It includes tirzepatide injections (Mounjaro) – a first in class long-acting dual glucose-dependent insulinotropic polypeptide GIP – as well as once-daily semaglutide tablets (Rybelsus), the notification said.
NICE recommended tirzepatide for patients with difficult to manage type 2 diabetes last year after taking into account additional data and modelling on cost effectiveness from manufacturers Eli Lilly.
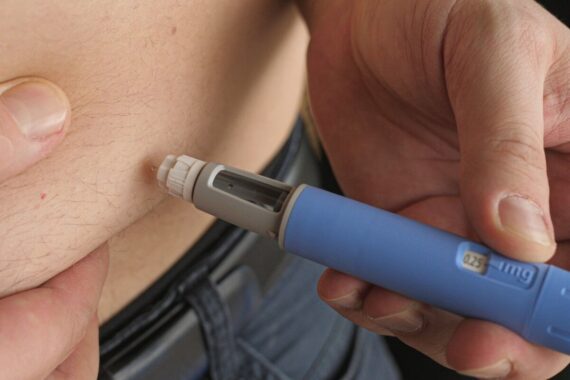
Patients with type 2 diabetes currently prescribed semaglutide (Ozempic) 0.25mg, 0.5mg and 1mg injections should be considered for treatment with semaglutide tablets or tirzepatide injections if they cannot obtain their medication for more than two weeks, the guidelines produced in collaboration with the Association of British Clinical Diabetologists said.
Liraglutide 6mg/ml solution is fully out of stock until the end of the year and if still clinically appropriate patients should be moved to the alternatives, it stated.
One and off supply issues for dulaglutide 0.75mg, 1.5mg, 3mg and 4.5mg injections are also expected in the coming months so if patients cannot get their medicine for a fortnight they should also be considered for a switch, it said.
The updated advice sets out the mode of action, side effects and contraindications for tirzepatide injections and semaglutide tablets to aid prescribers who may not have experience with them as well as practical advice for patients on how to take them.
It reiterates that clinicians should only prescribe the medicines for their licensed indication and ‘proactively engage with’ and prioritise for review those patients who have been impacted by the shortages which have been happening since last summer.
Prescribers should avoid doubling up lower dose preparations when higher doses are not available, switching between strengths based on availability or provide more than one month’s supply ‘unless there is a clear reason to do so’.
Local supply chain issues may be overcome by going to another pharmacy which should be considered before switching therapies, the guidance added.
‘It may now be appropriate to switch people with type 2 diabetes to an alternative GLP-1 RA or GIP/GLP-1 RA who are unable to obtain supply of their usual GLP-1 RA medication and who still meet the continuation criteria as outlined in NICE,’ the guidance said.
‘A clinical review of the patient case should still take place prior to consideration of switching where a clinical decision should be made as to whether a GLP-1 RA or GIP/GLP-1 RA is the most appropriate choice in the circumstances. There may be an alternative therapy more appropriate, or the possible need for insulin.’
Pulse October survey
Take our July 2025 survey to potentially win £1.000 worth of tokens




 Oviva’s fully remote Tier 3 Weight Management programme
Oviva’s fully remote Tier 3 Weight Management programme








