MHRA licenses tirzepatide for weight loss treatment in UK
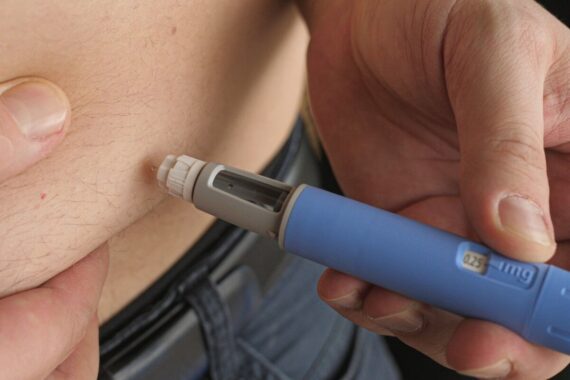
Type 2 diabetes treatmen tirzepatide has now been licensed in the UK for weight loss, the medicines regulator announced.
The Medicines and Healthcare products Regulatory Agency (MHRA) has agreed the new indication for tirzepatide (Mounjaro, produced by Eli Lilly) for adults aged 18 and over.
Under the licensed indication, the drug can be used for adults with a BMI of 30kg/m² or more as well as those with a BMI between 27-30kg/m² who also have weight-related health problems such as prediabetes, high blood pressure, high cholesterol, or heart problems.
NICE is currently reviewing the use of tirzepatide for weight loss on the NHS with guidance expected in March next year.
It follows a decision in March to recommend semaglutide (Wegovy) to be offered as part of NHS weight loss services. The first supplies were made available in September after reports of global shortages impacting diabetes patients.
The MHRA decision on tirzepatide was based on data from two international, randomised clinical trials, SURMOUNT-1 and SURMOUNT-2, that included patients with and without diabetes, which reported significant weight loss compared with placebo.
The impact of the treatment was dependent on dose but adults taking tirzepatide were found to have an average change in weight of between 16% and 22% compared with 2.4% for placebo in the first trial.
In SURMOUNT-2 which was for obese or overweight partipants who also had type 2 diabetes up to 86% of patients lost at least 5% of their body weight compared with 28% of those taking placebo.
The drug, which is given once a week in the form of pre-filled injections ranging from a starting dose of 2.5mg which can be increased over time to 15mg.
A dual receptor agonist, it acts on both glucose-dependent insulinotropic polypeptide (GIP) receptors and glucagon-like peptide-1 (GLP-1) receptors.
Tirzepatide should be used in conjunction with a reduced calorie diet and increased physical activity, the MHRA said, who added they would be keeping the safety of the drug under close review.
The regulator also warned prescribers and patients that patients using oral contraceptives should consider also using a barrier method of contraception or switching to a non-oral contraceptive method for four weeks after starting tirzepatide and for four weeks after each increase in dose.
NICE recommended tirzepatide for use in people with difficult to treat diabetes in NICE guidance published last month.
Initially rejected as an alternative to other antidiabetic drugs such as semaglutide and liraglutide, the committee changed its mind on use in specific circumstances after reviewing new evidence.
Julian Beach, MHRA interim executive director, healthcare quality and access, said: ‘We have prioritised rapid assessment of this new indication for Mounjaro, given the public health importance of access to new medicines to help tackle obesity.
‘We have drawn on advice from the independent Commission on Human Medicines in coming to our decision, and as with all products, will keep the safety of Mounjaro under close review.’
Health and Social Care Secretary Steve Barclay said: ‘Although further approvals are needed to use this in the NHS, Mounjaro has the potential to help thousands of people living with obesity and support those suffering from weight-related illnesses – if used alongside diet and physical activity.
‘Tackling obesity could help cut waiting lists and save the NHS billions of pounds’
Pulse July survey
Take our July 2025 survey to potentially win £1.000 worth of tokens

Visit Pulse Reference for details on 140 symptoms, including easily searchable symptoms and categories, offering you a free platform to check symptoms and receive potential diagnoses during consultations.
Related Articles
READERS' COMMENTS [1]
Please note, only GPs are permitted to add comments to articles












These multiple announcements to the media of miracle weight loss fat jabs are all very well, but yet again GPs are being left in the dark on exactly how this can all be delivered.
We’re told not to initiate without referral to weight-management clinics, who then tell disappointed punters that they can’t prescribe neither and to “go back to their GP”.
Meanwhile there’s chaos at the Chemist as diabetics are told their GLP1 supply has dried up, mainly because wealthier obese patients are gobbling up supplies privately.
The launch of GLP1 for non-diabetic obesity has been yet another car crash,. The desire to splash the “good news” across the media (without informing GPs in advance with clear protocols and plentiful supplies of the drugs) is causing totally predictable infuriation on both sides of the doctor’s desk.
Here’s a thought. How about informing Primary Care that a new treatment will be announced in a month’s time, precisely what the indications/contraindications are, who can have it, the pathway needed, and with clinics primed and ready to accept our referrals.
Is that really too much to ask???