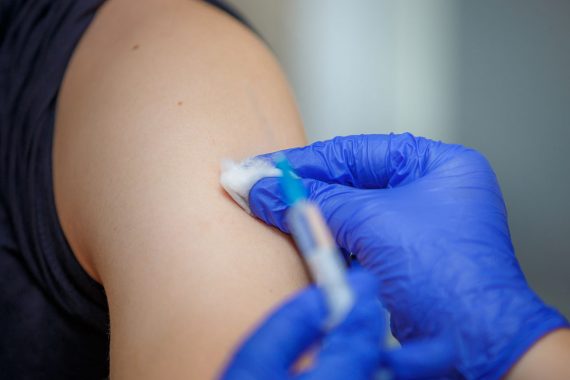
‘Millions’ more doses of flu vaccine are to be delivered after new laws have enabled the Government to distribute a yet-to-be-licensed vaccine.
Pulse reported in August that the Government was looking at Flublok – a quadrivalent influenza vaccine from Sanofi that has been used widely in the US – as a means to boost vaccine supplies.
Today, the Department of Health and Social Care said the MHRA has granted Flublok a ‘temporary license’ to be used in the UK.
This has been made possible by new laws that came into force on Friday last week, which also mean MHRA will be able to approve an unlicensed Covid-19 vaccine for UK use.
The DHSC said the vaccine has ‘met the standards of safety, quality and effectiveness’.
Deputy Chief Medical Officer Professor Jonathan Van Tam said: ‘As we approach the winter and cases of Covid-19 continue to rise, it is crucial we double down on efforts to vaccinate as many people as possible from flu.
‘We have increased the number of people eligible for free flu jabs this year to reduce all avoidable risks and protect people from illness.
‘Flublok has been in regular use in the United States – and the evidence shows that it is an excellent product.
‘I want to reassure everyone that all vaccines have undergone robust clinical trials and rigorous checks by the regulator to ensure they are safe, effective and of a high quality.’
It comes as the Government is targeting 30 million people in this year’s expanded flu vaccination campaign, with a larger number of patients having been made eligible for free flu jabs.
GPs in England have been able to place orders for additional flu vaccines from the Government’s central stock since last week. However, practices were advised they cannot use the Government’s central flu stock to ‘plug temporary shortfalls’ and must ‘exhaust’ other supply routes first.
Meanwhile, Northern Ireland’s Public Health Agency announced a ‘pause’ to all flu vaccinations for people under 65 this week – including those who are eligible for free jabs.
It said this comes as a result of ‘worldwide shortages’ of vaccine, which means ordering is not expected to resume until ‘mid-November’.
Pulse October survey
Take our July 2025 survey to potentially win £1.000 worth of tokens












As we are entering a new era of lockdown and continuing physical distancing measures for the foreseeable time is there really a need for a Flu vaccination campaign at all??
I agree with Neil Tallant.
Hopefully we will see a lot less flu this winter, but we’ll never know if it is due to a larger than usual campaign or just the COVID prevention measures which were responsible.
Also, regardless of the licensing issue, not all flu vaccs are the same. Is flublok active against the same strains of flu as we are likely to get in the UK this winter? I also think sme of the vaccine has been wasted – just been speaking to a 35 year old man with no health problems at all who was called by his practice for a flu jab. He has used efudix on some scalp lesions recently, so flagged up as being on chemotherapy!!